

This effect can be concentrated locally to form a pit, or crack. When metal atoms are exposed to an environment containing water molecules, they can give up electrons, becoming positively charged ions, provided an electrical circuit can be completed. An atom's electric charge dictates how molecules react with each other and in nature. In an electrical circuit, electric current is the movement of electrical charge. In a galvanic cell, positively charged ions flow to the cathode, while negative ions flow to the anode. Metal loss at anodic sites in an electrochemical cell occurs when the metal atoms give up one or more electrons and move as positively charged ions into the electrolyte. How do you know if an ion is present The charge of the element should always be represented beside the symbol if it is an ion. What are the two types of ion There are two types of ions : cations. Most metals become cations when they make ionic compounds. In a battery, the positive end (or cathode) is attractive to electrons, due to its positive charge. Positively charged ions are called cations. A positive charge also can be created by removing electrons from a neutrally charged object. A positive charge may be created by adding protons to an atom or object with a neutral charge. A metal reacts with a nonmetal to form an ionic bond.Ī positive charge occurs when the number of protons exceeds the number of electrons. The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound, like sodium chloride. Positively charged substances are repelled from other positively charged substances, but attracted to negatively charged substances. Lithium-ion batteries power everything from your mobile phone to your laptop.There are two types of electric charges: positive and negative. Negative ions in the body relax you and energize your body. Ions are sources of energy, whether in the human body or electrical gadgets. Some examples of molecular ions are NH 4 + (ammonium), OH – (hydroxide), etc. Similar to an atomic ion, a molecular ion may be molecular cation or anion.

When a molecule (a group of atoms bonded together) combines with an atomic ion, the molecule gets charged and becomes an ion. So atoms find it easier to lose or gain electrons to become ions. Protons are 1836 times heavier than electrons. Atom can gain of proton to get positively charged.Atom can lose a proton to get negatively charged.Atom can gain an electron to get negatively charged.Atom can lose an electron to get positively charged.There are 4 ways in which an atom can become an ion: This negatively charged ion is called anion. When an atom has more electrons than protons, it gets negatively charged. This positively charged ion is called cation. When an atom has more protons than neutrons, it gets positively charged. When the number of these charged particles is the same, the atom is neutral. So atoms are made up of charged particles (and also neutral ones). The protons and neutrons form the nucleus of the atom whereas electrons orbit around the nucleus, as shown in Figure 1.įigure 1: Electrons Revolving Round the Nucleus they have neither negative nor positive charge. Electrons are negatively charged while protons are positively charged. Sub-atomic particlesĪtom is made up of electrons, protons and neutrons.
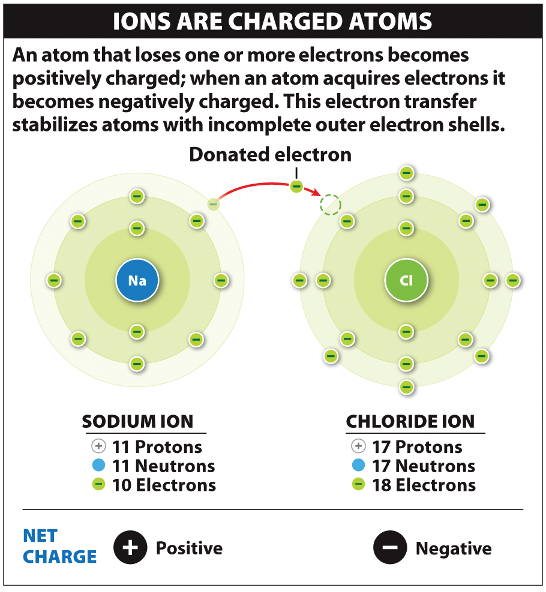
Alternately, we can say that atom is the smallest unit of matter that can exist in a stable form. As we discussed in a previous post, all matter is made up of atoms. Ions are nothing but atoms or molecules with positive or negative electrical charge.


 0 kommentar(er)
0 kommentar(er)
